Focused Photonics (Hangzhou) Inc. delivers innovative Pharmaceutical Manufacturing Solutions with precision Gas Analyzers and Process Monitoring Systems. Our technologies ensure purity control, contamination detection, and regulatory compliance in drug production, enhancing product quality and streamlining manufacturing processes for global pharmaceutical leaders.

The pharmaceutical manufacturing industry faces critical challenges amid rising demand and stringent regulations.
Narrative Insight: Unmonitored VOC and residual solvents in production environments lead to 5-10% batch rejections. The 2023 FDA recall of a major antibiotic due to contamination underscores the need for real-time monitoring.



Current trends emphasize real-time analytics and Industry 4.0, but reliance on traditional methods limits scalability and innovation.
|
Metric
|
Current State
|
Target
|
|---|---|---|
|
Purity Level |
95% |
99.9% |
|
Batch Rejection |
8% |
<2% |
Inconsistent process monitoring hampers achieving these goals.
FPI’s Pharmaceutical Manufacturing Solutions provide continuous gas analysis and contamination tracking with Laser-Based Analyzers. We ensure purity levels above 99.8% and reduce batch rejections by 75%, integrating with MES for seamless data integrity. Our systems support predictive maintenance, cutting downtime by 18%.
We tailor our expertise to your production needs
Conduct risk assessments and cleanroom audits.
Design Process Monitoring Systems for specific drug formulations.
Deploy with GMP-compliant installation in 5-7 weeks.
Provide 24/7 technical support, annual calibrations, and a 3-year warranty.
Our offerings blend innovative technology with actionable insights
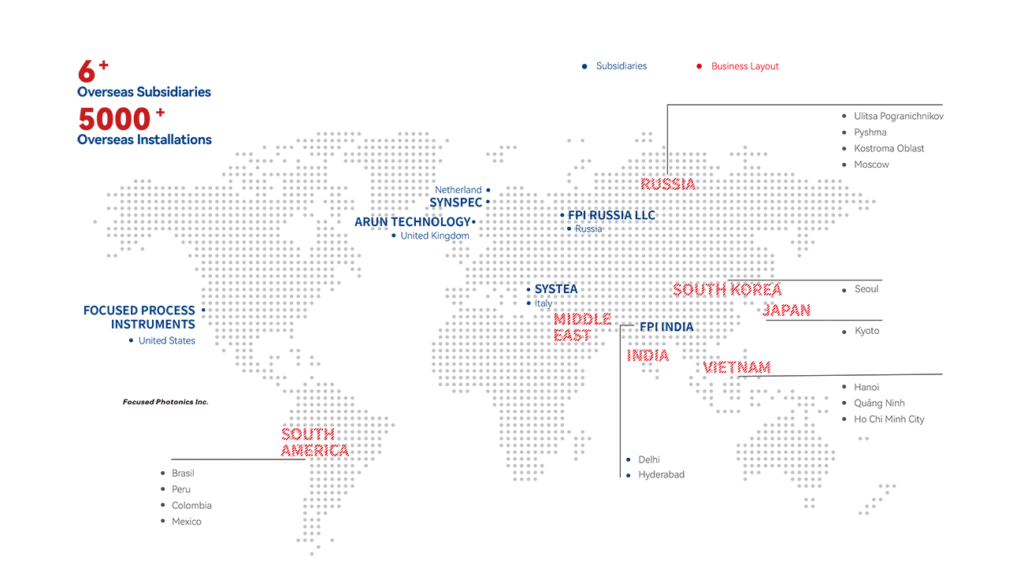
With 18+ years in pharma analytics, FPI has installed 250+ systems globally, achieving 98% customer satisfaction. Our laser technology ensures unmatched purity. Partner with us for
Contact Us
If you have any questions concerning our services, please fill out the contact form below.